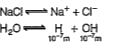A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ARIHANT PUBLICATION JHARKHAND-ELECTROCHEMISTRY, ACIDS , BASES , SALT AND HYDROLYSIS -EXAM BOOSTER FOR CRACKING EXAM
- Faraday's laws of electrolysis are related to
Text Solution
|
- An apparatus used for the measurement of quantity of electricity is kn...
Text Solution
|
- When sodium chloride solution is electrolysed , the gas that is l...
Text Solution
|
- The number of moles of oxygen obtained by the electrolytic decompositi...
Text Solution
|
- The unit of electro-chemical equivalent is
Text Solution
|
- To deposit 0.6354 g of copper by electrolysis of aqueous cupric sulpba...
Text Solution
|
- The cathodic reaction in electrolysis of dilute H(2)SO(4) with platinu...
Text Solution
|
- One Faraday of electricity will liberate 1g atom of the metal from the...
Text Solution
|
- If the current is passed into the solution of the electrolyte
Text Solution
|
- A certain current liberated 0.504 g of hydrogen in 2 hours. How many g...
Text Solution
|
- The atomic weight of silver and copper are 108 and 64. A silver voltam...
Text Solution
|
- The best conductor of electricity in 0.01 M solution of
Text Solution
|
- A current of 'c' amperes is passed through the solution of an electrol...
Text Solution
|
- According to Faraday's second law of electrolysis
Text Solution
|
- An electrolytic cell contains aqueous solution of silver sulphate and ...
Text Solution
|
- When an electric current is passed through an aqueous solution of sodi...
Text Solution
|
- A solution of sodium sulphate in water is electrolysed using inert ele...
Text Solution
|
- Sodium cannot be extracted by electrolysis of brine because
Text Solution
|
- During electrolysis, the process taking place at cathode is
Text Solution
|
- The reference electrode is made from one of the following
Text Solution
|