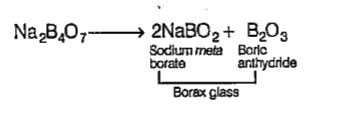A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ARIHANT PUBLICATION JHARKHAND-IMPORTANT CHEMICAL COMPOUNDS-Exam Booster for Cracking Exam
- How many molecules of water of crystallisation are present in blue vit...
Text Solution
|
- How many molecules of water are there in the formula of simple alum ?
Text Solution
|
- On strong heating of borax, a transparent glassy mass is obtained as a...
Text Solution
|
- The gas produced by the action of dil, sulphuric acid on bleaching pow...
Text Solution
|
- The formula of alum is
Text Solution
|
- The molecular formula of bleaching powder is
Text Solution
|
- The formula of caustic soda is
Text Solution
|
- The chemical formula of marble is
Text Solution
|
- The chemical formula of lime is
Text Solution
|
- The chemical name of blue vitriol is
Text Solution
|
- The molecular formula of blue vitriol is
Text Solution
|
- The chemical formula of borax is
Text Solution
|
- The chemical name of borax is
Text Solution
|
- Bleaching powder becomes useless on exposure to air because
Text Solution
|
- The compounds among the following, used as disinfectants or germicides...
Text Solution
|
- Red drug is
Text Solution
|
- The chemical formula of salt petre is
Text Solution
|
- The chemical name of salt petre is
Text Solution
|
- Red lead is
Text Solution
|
- The molecular formula of litharge is
Text Solution
|