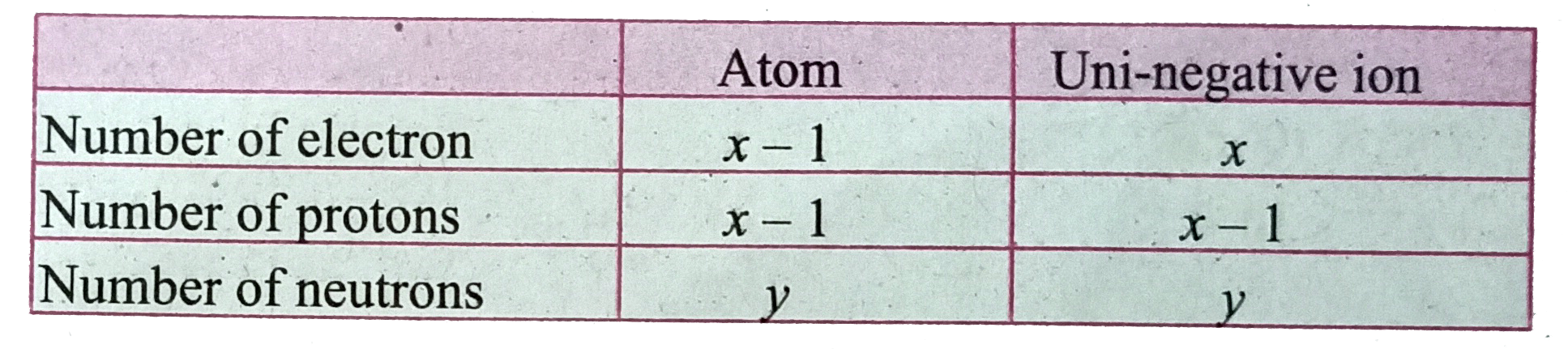
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- An ion with mass number 37 possesses unit negative charge. If the ion ...
Text Solution
|
- An ion with mass number 37 possesses one unit off negative charge. If ...
Text Solution
|
- An ion with mass number 56 contains 3 units of positive charge and 30....
Text Solution
|
- 37 द्रव्यमान संख्या वाले एक आयन पर ऋणावेश की एक इकाई है। यदि आयन में इ...
Text Solution
|
- An ion with mass number 37 posseses one unit of negative charge. If th...
Text Solution
|
- 37 द्रव्यमान संख्या वाले एक आयन पर ऋणावेश की एक इकाई है। यदि आयन में इ...
Text Solution
|
- Mass number of an ion with unit negative charge is 37. Number of neutr...
Text Solution
|
- An ion with mass number 37 possesses one unit off negative charge. If ...
Text Solution
|
- An ion with mass number 37 prossesses unit negative charg. If contains...
Text Solution
|