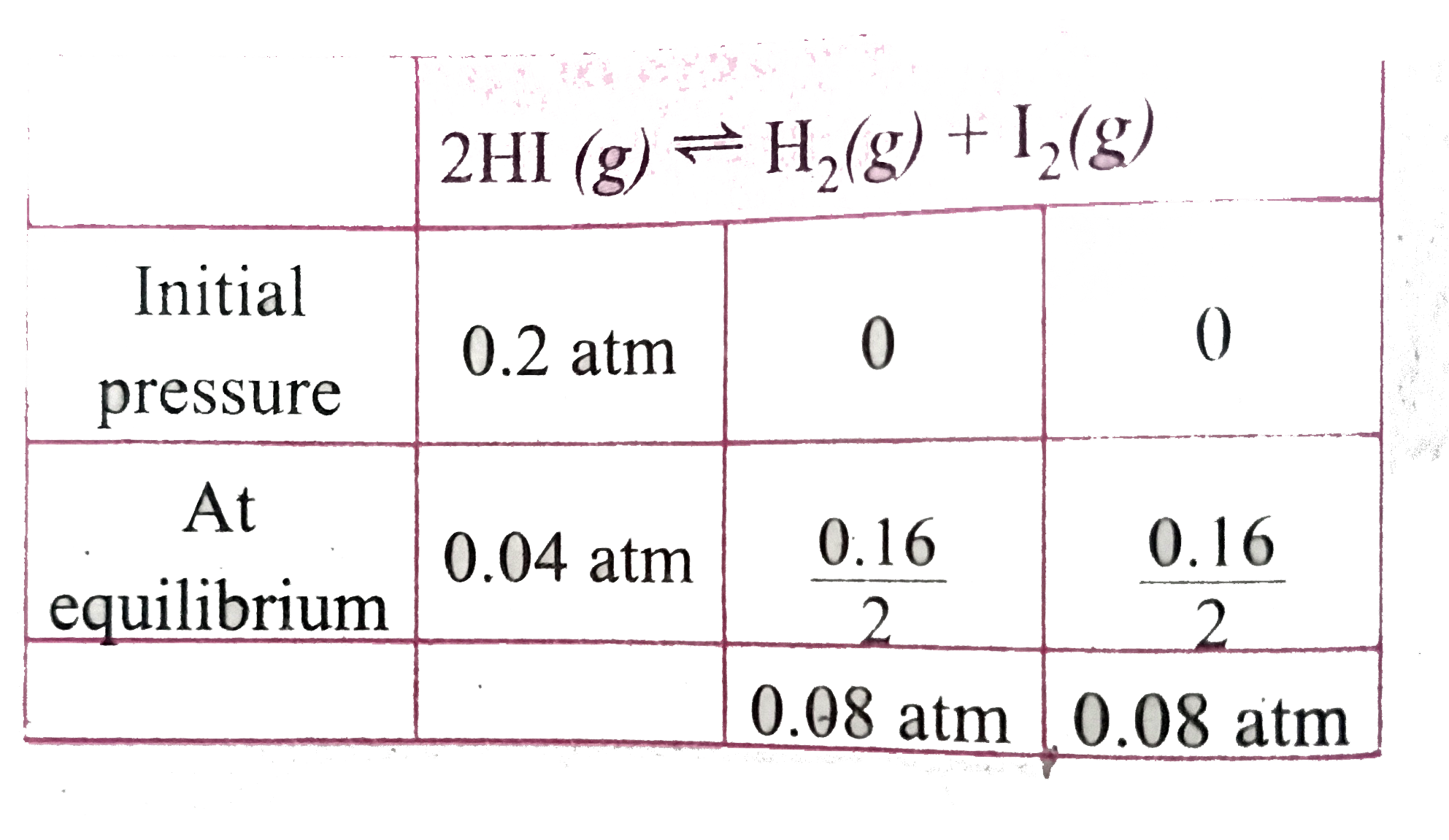A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A sample of HI(g) is placed in a flask at a pressure of 0.2 atm. At eq...
Text Solution
|
- A sample of HI(g) is placed in flask at a pressure of 0.2 atm. At equi...
Text Solution
|
- Calculate partial pressure of B at equilibrium in the following equili...
Text Solution
|
- A sample of HI(g) is placed in flask at a pressure of 0.2 atm. At equi...
Text Solution
|
- HI(g) का एक नमूना 0.2 atm दाब पर एक फ्लक्स में रखा जाता है। साम्य पर H...
Text Solution
|
- एक HI((g)) का सैम्पल 0.2 वायुमण्डल दाब पर फ्लास्क में रखा गया | साम्...
Text Solution
|
- A sample of HI(g) is placed in flask at a pressure of 0.2 atm. At equi...
Text Solution
|
- A sample of HI(g) is placed in flask at a pressure of 0.2atm. At equil...
Text Solution
|
- A sample of HI (g) is placed in a flask at a pressure of 0.2atm. In ca...
Text Solution
|