Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Sodium hydroxide and HCl acid react as shown in this equation NaOH((...
Text Solution
|
- Calculate the volume of 1.00 mol L^(-1) aqueous sodium hydroxide that ...
Text Solution
|
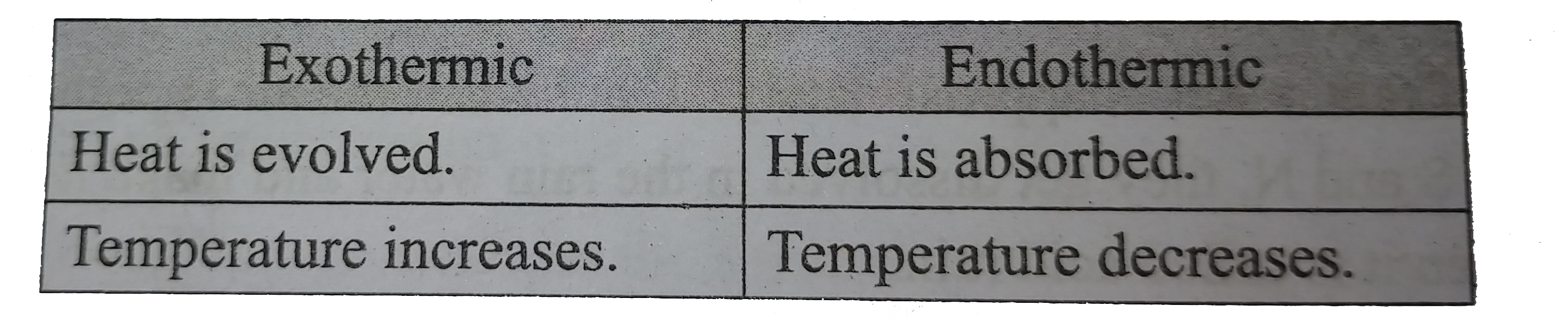
- Differentiate between Exothermic And Endothermic Reactions
Text Solution
|
- Complete the following chemical reaction equations : (i)P (4(s)) +Na...
Text Solution
|
- What happens when an acid reacts with a base? Explain by taking the ex...
Text Solution
|
- The reaction NaOH((s)) + H(2)O((I)) to NaOH((aq)) is a/an ………… reactio...
Text Solution
|
- Write the balanced chemical equations for the following and identify t...
Text Solution
|
- Differentiate exothermic and endothermic reactions.
Text Solution
|
- What are exothermic and endothermic chemical reactions?
Text Solution
|