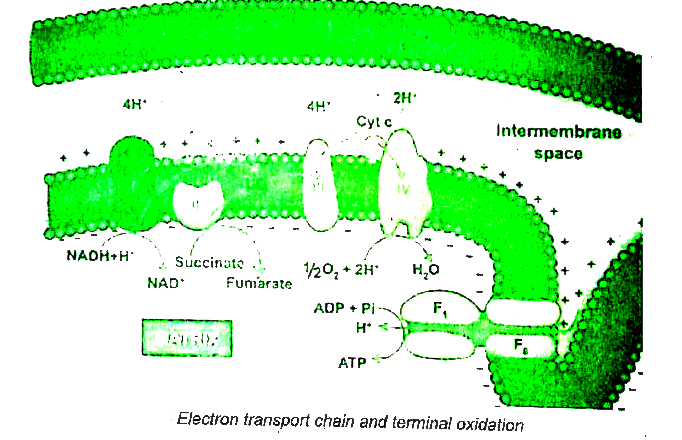
During glycolysis, link reaction and Krebs cycle the respirator substrates are oxidised at several steps and as result many reduced coenzymes `NADH + H^(+)` and `FADH_(2)` are produced. These reduced coenzymes are transported to inner membrane of mitochondria and are converted back to their oxidised forms produce electrons and protons. In mitochondria, the inner membrane is folded in the form of finger projections towards the matrix celled cristae. In cristae many oxysomes (`F_(1)` particles) are present which have electron transport carriers are present. According to Peter Mitchell.s Chemiosmotic theory this electron transport is coupled to ATP synthesis. Electron and hydrogen (proton) transport takes place across four multiprotein complexes (I-IV). The are
(i) Complex-I (NADH dehydrogenase: It contains a flavoprotein (FMN) and associated with non-heme iron Sulphur protein (Fe-S). This complex is responsible for passing electrons and protons from mitochondrial NADH (Internal) to Ubiquinone (UQ).
`NADH+H^(+) UQ hArr NAD^(+) +UQH_(2)`
In plants, an additional NADH dehydrogenase (External) complex is present on the outer surface of inner membrane of mitochondria which can oxidise cytosolic `NADH + H^(+)`. Ubiquinone (UQ) or Coenzyme Quinone (Co Q) is a small, lipid soluble electron, proton carrier located within the inner membrane of mitochondria.
(ii) Complex-II (Succinic dehydrogenase): It contains FAD flavoprotein is associated with non-heme iron Sulphur (Fe-S) protein. from succinate in Krebs cycle and is converted into fumarate and passes to ubiquinone.
Succinate `+UQrarr" Fumarate" +UQH_(2)`
(iii) Complex-III (Cytochrome `bc_(1)` complex):
This complex oxidises reduced ubiquinone (ubiquinol) and transfers the electrons throgh Cytochrome `bc_(1)` Complex (Iron Sulphur center `bc_(1)` complex) to cytochrome c. Cytochrome c is a small protein attached to the outer surface of inner membrane and act as a mobile carrier to transfer electrons between complex III to complex IV.
`UQH_(2) + 2Cyt C_(" oxidised") hArr UQ + 2Cyt c _("reduced ")+2H^(+)`
(iv) Complex IV (Cytochrome c oxidase):
This complex contains two copper centers (A and B) and cytochromes a and `a_(3)`. Complex IV is the terminal oxidase and bring about the reduction of `1/2 O_(2) to H_(2)O`. Two protons are needed to form a moleule of `H_(2)O` (terminal oxidation).
` 2Cyt C _("oxidised ")+ 2H^(+) + 1//2 O_(2) hArr 2Cyt C_("reduced ") +H_(2)O`
The transfer of electrons from reduced coenzyme NADH to oxygen via complexes I to IV is coupled to the synthesis of ATP from ADP and inorganic phosphate (Pi) which is called Oxidative phosphorylation. The `F_(0)F_(1)` - ATP synthase (also called complex V) consists of `F_(0) and F_(1)`. `F_(1)` converts ADP and Pi to ATP and is attached to the matrix side to the inner membrane. `F_(0)` is present in inner membrane and acts as a channel through which protons come into matrix.
Oxidation of one molecule of `NADH + H^(+)` gives rise to 3 molecules of ATP and oxidation of one molecule of `FADH_(2)` produces 2 molecules of ATP within a mitochondrion. But cytoplasmic `NADH + H(^(+)` yields only two ATPs though external NADH dehydrogenase. Therefor, two reduced coenzyme (`NADH + H^(+)`) molecules from glycolysis being extra mitochondrial will yicld `2xx3=4` ATP molecules instead of 6 ATPs. The Mechanism of mitochondrial ATP synthesis is based on Chemiosmotic hypothesis. According to this theory electron carriers present in the inner mitochondrial membrance allow for the transfer of protons `(H^(+))`. For the production of single ATP, 3 protons `(H^(+))` are needed. The terminal oxidation of external NADH bypasses the first phosphorylation site and hence only two ATP molecules are produced per external NADH oxidised through However, in those animal tissues in which malate shuttle mechanism is present, the oxidation of external NADH will yield almost 3 ATP molecules.
Complete oxidation of a glucose molecule in aerobic respiration result in the net gain of 36 ATP molecules in plants. Since huge amount of energy is generated in mitochondria in the form of ATP molecules they are called .power house of the cell.. In the case of aerobic prokaryotes due to lack of mitochondria each molecule of glucose produces 38 ATP molecules.