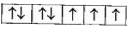
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STRUCTURE OF ATOMS
BRILLIANT PUBLICATION|Exercise LEVEL - III (Single Correct Answer Type)|9 VideosSTRUCTURE OF ATOMS
BRILLIANT PUBLICATION|Exercise LEVEL - III (Multiple Correct Answer Type)|14 VideosSTRUCTURE OF ATOMS
BRILLIANT PUBLICATION|Exercise LEVEL - II (ASSERTION - REASON)|5 VideosSTATES OF MATTER
BRILLIANT PUBLICATION|Exercise LEVEL III (Linked Comprehension Type )|9 VideosTHERMODYNAMICS AND CHEMICAL ENERGETICS
BRILLIANT PUBLICATION|Exercise (LEVEL-II (HOMEWORK)|39 Videos
BRILLIANT PUBLICATION-STRUCTURE OF ATOMS-LEVEL - I
- 'Heisenberg's uncertainty principle can be expressed as
Text Solution
|
- Calculate the wavelength (in nanometer) associated with a proton movin...
Text Solution
|
- The total spin resulting from a d^(7) configuration is
Text Solution
|
- An ion that has 18 electrons in the outermost shell is
Text Solution
|
- The wavelength associated with a golf ball weighing 300 g and moving a...
Text Solution
|
- Which of the following pairs is isodiaphers?
Text Solution
|
- In Bohr’s stationary orbits
Text Solution
|
- The distance between 4^(th) and 3^(rd) Bohr orbits of He^(+) is :
Text Solution
|
- Line spectra is characteristic of:
Text Solution
|
- With increasing principal quantum number, the energy difference betwee...
Text Solution
|
- The total number of orbitals in M shell of an atom is
Text Solution
|
- Which of the following statements is correct?
Text Solution
|
- The number of unpaired electrons in ""(27)Co is
Text Solution
|
- The numbers of spherical and angular nodes in 4f orbitals, respectivel...
Text Solution
|
- Which of the following sets represents isoelectronic species?
Text Solution
|
- The electronic configuration of ""(24)Cr is
Text Solution
|
- The number of possible orientations of d orbitals in space is
Text Solution
|
- Which electronic configuration does not follow the Aufbau rule? : 1s...
Text Solution
|
- The subshells which are filled just before and just after the filling ...
Text Solution
|
- The magnetic moment and nature for isolated gaseous ion Au^(3+) is. :...
Text Solution
|