A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STRUCTURE OF ATOMS
BRILLIANT PUBLICATION|Exercise LEVEL - II (ASSERTION - REASON)|5 VideosSTRUCTURE OF ATOMS
BRILLIANT PUBLICATION|Exercise LEVEL - I|107 VideosSTRUCTURE OF ATOMS
BRILLIANT PUBLICATION|Exercise LEVEL - III (Linked Comprehension Type)|8 VideosSTATES OF MATTER
BRILLIANT PUBLICATION|Exercise LEVEL III (Linked Comprehension Type )|9 VideosTHERMODYNAMICS AND CHEMICAL ENERGETICS
BRILLIANT PUBLICATION|Exercise (LEVEL-II (HOMEWORK)|39 Videos
BRILLIANT PUBLICATION-STRUCTURE OF ATOMS-LEVEL - II
- A 600 W mercury lamp emits monochromatic radiation of wavelength 331.3...
Text Solution
|
- Which one of the following is not the characteristic of Planok's quant...
Text Solution
|
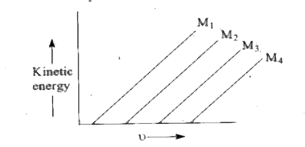
- A plot of the kinetic energy ((1)/(2) mv^(2)) of ejected electrons as ...
Text Solution
|
- The highest energy in Balmer series, in the emission spectra of hydrog...
Text Solution
|
- If the electronic configuration of nitrogen had 1s^(7), it would have ...
Text Solution
|
- An excited He^(+) ion emits photon of wavelength lamda in returning to...
Text Solution
|
- If the energy of H-atom in the ground state is-E, the velocity of phot...
Text Solution
|
- The energy of I, II and III energy levels of a certain atom are E, (4E...
Text Solution
|
- An electron in a hydrogen atom in its ground state absorbs 1.5 times a...
Text Solution
|
- An alpha-particle having kinetic energy 5 MeV falls on a Cu-foil. The ...
Text Solution
|
- The energy of separation of an electron in a Hydrogen like atom in exc...
Text Solution
|
- Photons of frequency, v, fall on metal surface for which the threshold...
Text Solution
|
- When electron jumps from the fourth orbit to the second orbit in Het i...
Text Solution
|
- Which of the following graph represents the radial probability functio...
Text Solution
|
- For an atom or ion having single electron, compare the energies of the...
Text Solution
|
- Excited hydrogen atom emits light in the ultra violet region at 2.47 x...
Text Solution
|
- Which of the following statements on quantum numbers is not correct?
Text Solution
|
- The electrons, identified by quantum numbers 'n' and (i) 'n=4, l=1' (i...
Text Solution
|
- The kinetic and potential energy (in eV) of an electron present in 3^(...
Text Solution
|
- The stopping potential of the electrons emitted in a photoelectric exp...
Text Solution
|