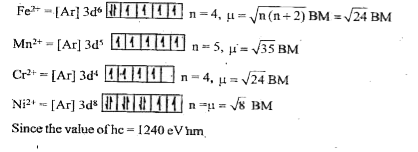Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STRUCTURE OF ATOMS
BRILLIANT PUBLICATION|Exercise LEVEL - III (Matching Column Type)|5 VideosSTRUCTURE OF ATOMS
BRILLIANT PUBLICATION|Exercise LEVEL - III (StatementType)|5 VideosSTRUCTURE OF ATOMS
BRILLIANT PUBLICATION|Exercise LEVEL - III (Multiple Correct Answer Type)|14 VideosSTATES OF MATTER
BRILLIANT PUBLICATION|Exercise LEVEL III (Linked Comprehension Type )|9 VideosTHERMODYNAMICS AND CHEMICAL ENERGETICS
BRILLIANT PUBLICATION|Exercise (LEVEL-II (HOMEWORK)|39 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-STRUCTURE OF ATOMS-LEVEL - III (Numerical type)
- Find the quantum no: 'n' corresponding to the excited state of He+ ion...
Text Solution
|
- The wavelength of m^(th) line Balmer series for an orbital is 4103Å. T...
Text Solution
|
- Ionisation potential of hydrogen atom is 13.6 eV. If ground state of H...
Text Solution
|
- If magnetic quantum number of a given electron in an atom is -3, then ...
Text Solution
|
- How many of the following ions have the same magnetic moments? Fe^(2+)...
Text Solution
|
- The work function (phi) of some metals is listed below. The number of ...
Text Solution
|
- Not considering the electronic spin, the degeneracy of the second exci...
Text Solution
|
- The maximum number of electrons that can have principal quantum number...
Text Solution
|
- A atomic masses of He and Ne are 4 and 20 amu respectively. The value ...
Text Solution
|