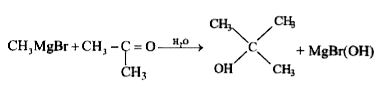
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALDEHYDES AND KETONES
BRILLIANT PUBLICATION|Exercise LEVEL-II|42 VideosALCOHOLS, PHENOLS AND ETHERS
BRILLIANT PUBLICATION|Exercise LEVEL III (Linked Comprehension Type) |12 VideosBIOMOLECULES, POLYMERS AND CHEMISTRY IN EVERYDAY LIFE
BRILLIANT PUBLICATION|Exercise LEVEL-II (ASSERTION-REASON TYPE QUESTIONS)|6 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-ALDEHYDES AND KETONES -LEVEL-II
- Grignard's reagent reacts with ethanal (acetaldehyde) and propanone to...
Text Solution
|
- The general formula of aliphatic saturated aldehydes and ketones is:
Text Solution
|
- The IUPAC name of the compound
Text Solution
|
- A single compound of the structure : OHC-CH(2)-overset(CH(3))overset(|...
Text Solution
|
- Predict the correct intermediate and product in the following reaction...
Text Solution
|
- The reaction temperature of the preparation of butanal by the oxidatio...
Text Solution
|
- The most suitable oxidising agent for the conversion of cyclohex-2-en-...
Text Solution
|
- Which one of the following on alkaline hydrolysis produces a ketone? ...
Text Solution
|
- The compound which is formed during the dry distillation of calcium be...
Text Solution
|
- X & Y are respectively
Text Solution
|
- The reaction by which benzaldehyde cannot be prepared is
Text Solution
|
- Which of the following pathways does not produce hexan-2-one?
Text Solution
|
- The major product of the following reaction is:
Text Solution
|
- In the following reaction: Aldehyde + Alcohol overset("Dry HCl")rarr A...
Text Solution
|
- The major product X formed in the following reaction is
Text Solution
|
- The reagent 'X' to be used for this conversion is
Text Solution
|
- The most suitable reagent (Y) for the following conversion is
Text Solution
|
- Under Clemmensen or Wolff-Kishner conditions of reduction, the convers...
Text Solution
|
- Compound 'X' (C(3)H(8)O) is treated with acidified K(2)Cr(2)O(7) to fo...
Text Solution
|
- Match the following:
Text Solution
|
- When pentan-2-one is treated with conc. HNO(3) at elevated temperature...
Text Solution
|