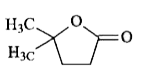
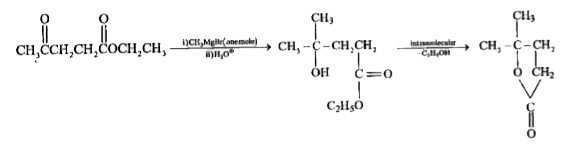
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- CH3 overset(O)overset(||)C CH2 CH2 overset(O)overset(||)C OCH2 CH3 ove...
Text Solution
|
- A monomer of a polymer on ozonolysis gives two moles of CH2O and one m...
Text Solution
|
- निम्न अभिक्रिया, CH3-underset(CH3)underset|overset(CH3)overset|C-O Na ...
Text Solution
|
- CH3-overset(CH3)overset|CH-CH2-O-CH2-CH3+HI overset"तापित"to में निम्...
Text Solution
|
- The IUPAC name of CH3 CH2 - overset(O ) overset(||)C-CH2-overset...
Text Solution
|
- The IUPAC name for CH3 - overset(O)overset(||)C - CH2 - CH2 -overset(O...
Text Solution
|
- CH3 - overset(CH3)overset(|)C-overset(O)overset(||)C - CH2 - CH2 OH य...
Text Solution
|
- The correct IUPAC name of CH3-overset(CH3)overset(|)overset(CH2)overse...
Text Solution
|
- CH3 - CH2 - overset(O ) overset(|| ) C - underset(CH3) underset(|) ove...
Text Solution
|