A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-STATES OF MATTER : GASES AND LIQUIDS-SINGLE CHOICE QUESTION
- An open vessel at 27 ^@C is heated until 3/8th of the air in it has be...
Text Solution
|
- The rates of diffusion of SO(2), CO(2), PCl(3) and SO3 are in the fol...
Text Solution
|
- The density of a gas-filled electric lamp is 0.75, after the lamp has ...
Text Solution
|
- The rms speed of N2 molecules in a gas is u. If the temperature is dou...
Text Solution
|
- A gas obeys the equation of state P(Vm – b) = RT. The slope of the iso...
Text Solution
|
- In the following reaction, we start with 2 mol of N2 and 5 mol of H2 e...
Text Solution
|
- The weight of 350 mL of a diatomic gas at 0^@C and 2 atm pressure is 1...
Text Solution
|
- For the given isotherm for one mole of an ideal gas, which follows Boy...
Text Solution
|
- An ideal gas is initially at temperature T and volume V. It's volume i...
Text Solution
|
- What is the correct relation between critical temperature Tc, Boyle's ...
Text Solution
|
- If the rate of diffusion of A is 5 times that of B, what will be the d...
Text Solution
|
- At what temperature will the molar kinetic energy of 0.3 mol of He be ...
Text Solution
|
- A mixture of dihydrogen and dioxygen at one bar pressure contains 20% ...
Text Solution
|
- Consider the following graph: X, Y and Z can be respectively.
Text Solution
|
- A sample of air contains only N(2), O(2) and H(2). It is saturated wi...
Text Solution
|
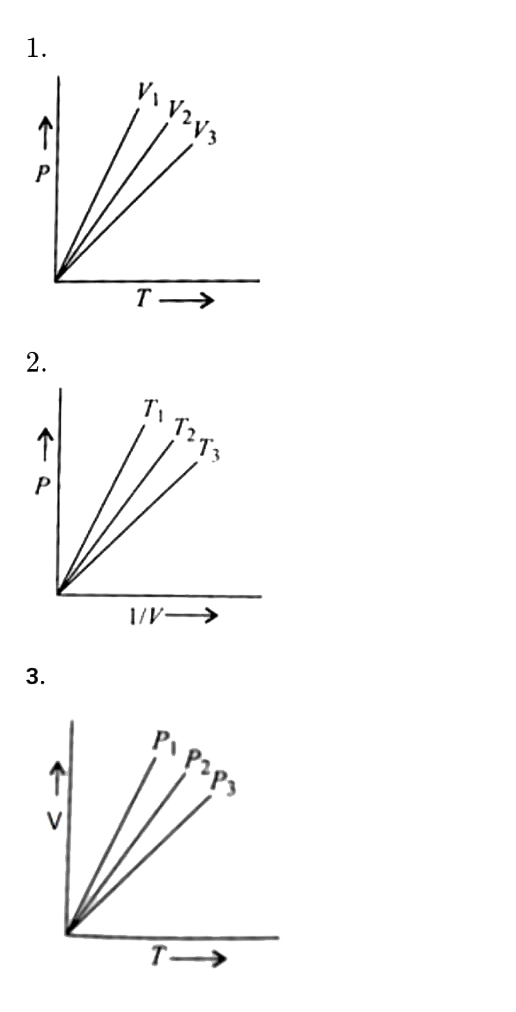
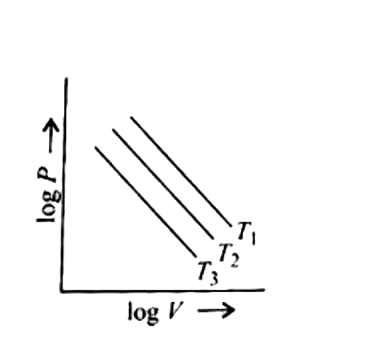
- For 1 mol of an ideal gas, V(1) gt V(2) gt V(3) in fig. 1, T(1) gt T(2...
Text Solution
|
- A gas can be compressed to a fraction of its volume. The same volume o...
Text Solution
|
- For Co, isotherm is of the type as shown. Near point A, compressibilit...
Text Solution
|
- The ratio of rates of diffusion of gases X and Y is 1 : 5 and that of ...
Text Solution
|
- Under which of the following conditions do real gases approach the ide...
Text Solution
|