Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BAL BHARTI-PERIODIC CLASSIFICATION OF ELEMENTS-Exercise
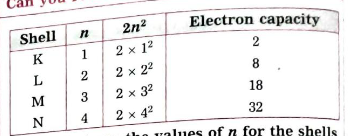
- What are the values of n for the shells K,L and M?
Text Solution
|
- Rearrange the columns 2 and 3 so as to match with the column 1:
Text Solution
|
- The number of electrons in the outermost shell of alkali metals is
Text Solution
|
- Alkali earth metals have valency 2.this means that their position in t...
Text Solution
|
- Molecular formula of the chloride of an element X is XCI. This compoun...
Text Solution
|
- In which block of the modern periodic table are the non-metlas present...
Text Solution
|
- An element has its electron configuration as 2,8,2. Answer the followi...
Text Solution
|
- An element has its electron configuration as 2,8,2. Answer the followi...
Text Solution
|
- An element has its electron configuration as 2,8,2. Answer the followi...
Text Solution
|
- An element has its electron configuration as 2,8,2. Answer the followi...
Text Solution
|
- Write down the electronic configuration of the following elements from...
Text Solution
|
- Write down the electronic configuration of the following elements from...
Text Solution
|
- Write down the electronic configuration of the following elements from...
Text Solution
|
- Write down the electronic configuration of the following elements from...
Text Solution
|
- Write down the electronic configuration of the following elements from...
Text Solution
|
- Write down the electronic configuration of the following elements from...
Text Solution
|
- Write down the electronic configuration of the following elements from...
Text Solution
|
- Write down the electronic configuration of the following elements from...
Text Solution
|
- The atom having the smallest size.
Text Solution
|
- The atom having the smallest atomic mass .
Text Solution
|
- Write the name and symbol of the element from the description. The m...
Text Solution
|