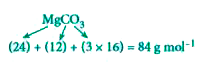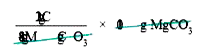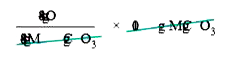Text Solution
Verified by Experts
Topper's Solved these Questions
BASIC CONCEPTS OF CHEMISTRY AND CHEMICAL CALCULATIONS
NCERT TAMIL|Exercise Evaluate Yourself|13 VideosBASIC CONCEPTS OF CHEMISTRY AND CHEMICAL CALCULATIONS
NCERT TAMIL|Exercise EVALUATION ( Choose the best answer) |25 VideosATOMIC STRUCTURE-I
NCERT TAMIL|Exercise QUESTIONS (D. EXPLAIN BRIEFLY ON THE FOLLOWING)|6 VideosBASIC CONCEPTS OF ORGANIC CHEMISTRY
NCERT TAMIL|Exercise QUESTION|22 Videos
Similar Questions
Explore conceptually related problems
NCERT TAMIL-BASIC CONCEPTS OF CHEMISTRY AND CHEMICAL CALCULATIONS-EVALUATION (Write brief answer to the following questions)
- Calculate the percentage composition of the elements present in magnes...
Text Solution
|
- The density of carbon dioxide is equal to 1.965 kgm^(-3) at 273 K and ...
Text Solution
|
- Which contains the greatest number of moles of oxygen atoms i) 1 mo...
Text Solution
|
- Calculate the average atomic mass of naturally occurring magnesium usi...
Text Solution
|
- In a reaction x + y + z(2) = xyz(2) identify the Limiting reagent if ...
Text Solution
|
- Mass of one atom of an element is 6.645 xx 10^(-23) g. How many moles ...
Text Solution
|
- What is the empirical formula of the following ? (i) Fructose (C(6)H...
Text Solution
|
- The reaction between aluminium and ferric oxide can generate temperatu...
Text Solution
|
- How many moles of ethane is required to produce 44 g of CO(2(g)) after...
Text Solution
|
- Hydrogen peroxide is an oxidising agent. It oxidises ferrous ion to fe...
Text Solution
|
- Calculate the empirical and molecular formula of a compound containing...
Text Solution
|
- A Compound on analysis gave Na = 14.31% S = 9.97% H= 6.22% and O= 69.5...
Text Solution
|
- Balance the following equations by oxidation number method K(2)Cr(2)...
Text Solution
|
- Balance the following equations by oxidation number method KMnO(4) +...
Text Solution
|
- Balance the following equations by oxidation number method Cu+HNO(3)...
Text Solution
|
- Balance the following equations by oxidation number method KMnO(4) +...
Text Solution
|
- Balance the following equations by ion electron method. KMnO(4) +SnC...
Text Solution
|
- Balance the following equations by ion electron method. C(2)O(4)^(2-...
Text Solution
|
- Balance the following equations by ion electron method. Na(2)S(2)O(3...
Text Solution
|
- Balance the following equations by ion electron method. Zn+NO(3)^(-)...
Text Solution
|