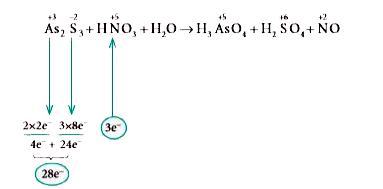
Text Solution
Verified by Experts
Topper's Solved these Questions
BASIC CONCEPTS OF CHEMISTRY AND CHEMICAL CALCULATIONS
NCERT TAMIL|Exercise EVALUATION ( Choose the best answer) |25 VideosBASIC CONCEPTS OF CHEMISTRY AND CHEMICAL CALCULATIONS
NCERT TAMIL|Exercise EVALUATION (Write brief answer to the following questions) |19 VideosBASIC CONCEPTS OF CHEMISTRY AND CHEMICAL CALCULATIONS
NCERT TAMIL|Exercise EVALUATION (Write brief answer to the following questions) |19 VideosATOMIC STRUCTURE-I
NCERT TAMIL|Exercise QUESTIONS (D. EXPLAIN BRIEFLY ON THE FOLLOWING)|6 VideosBASIC CONCEPTS OF ORGANIC CHEMISTRY
NCERT TAMIL|Exercise QUESTION|22 Videos
Similar Questions
Explore conceptually related problems
NCERT TAMIL-BASIC CONCEPTS OF CHEMISTRY AND CHEMICAL CALCULATIONS-Evaluate Yourself
- By applying the knowledge of chemical classification, classify each of...
Text Solution
|
- Calculate the molar mass of the following. Ethanol (C(2)H(5)OH)
Text Solution
|
- Calculate the molar mass of the following. Potassium permanganate (...
Text Solution
|
- Calculate the molar mass of the following. Potassium dichromate (K(...
Text Solution
|
- Calculate the molar mass of the following. Sucrose (C(12)H(22)O(11...
Text Solution
|
- Calculate the number of moles present in 9 g of ethane.
Text Solution
|
- Calculate the number of molecules of oxygen gas that occupies a volume...
Text Solution
|
- 0.456 g of a metal gives 0.606 g of its chloride. Calculate the equiva...
Text Solution
|
- Calculate the equivalent mass of potassium dichromate. The reduction h...
Text Solution
|
- A Compound on analysis gave the following percentage composition C=54....
Text Solution
|
- Experimental analysis of a compound containing the elements x,y,z on a...
Text Solution
|
- The balanced equation for a reaction is given below 2x+3y to 4l+m ...
Text Solution
|
- Balance the following equation using oxidation number method Ag(2)S(...
Text Solution
|