Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT TAMIL-QUANTUM MECHANICAL MODEL OF ATOM-EVALUATION (Write brief answer to the questions)
- An atom of an element contains 35 electrons and 45 neutrons. Deduce (...
Text Solution
|
- Calculate the energy required for the process. He((g))^(+) to He((g)...
Text Solution
|
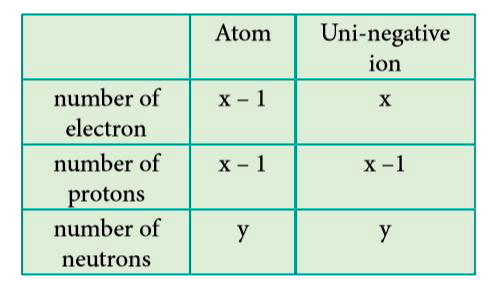
- An ion with mass number 37 possesses unit negative charge. If the ion ...
Text Solution
|
- The Li^(2+) ion is a hydrogen like ion that can be described by the Bo...
Text Solution
|
- Protons can be accelerated in particle accelerators. Calculate the wav...
Text Solution
|
- What is the de Broglie wavelength (in cm) of a 160g cricket ball trave...
Text Solution
|
- Suppose that the uncertainty in determining the position of an electro...
Text Solution
|
- Show that if the measurement of the uncertainty in the location of the...
Text Solution
|
- What is the de Broglie wave length of an electron, which is accelerate...
Text Solution
|
- Identify the missing quantum numbers and the sub energy level
Text Solution
|
- How many orbitals are possible for n =4?
Text Solution
|
- How many radial nodes for 2s, 4p, 5d and 4f orbitals exhibit? How many...
Text Solution
|
- Consider the following electronic arrangements for the d^(5) configura...
Text Solution
|
- Define orbital? what are the n and l values for 3p(x) and 4d(x^(2)-y^(...
Text Solution
|
- Calculate the uncertainty in position of an electron, if Deltav = 0.1%...
Text Solution
|
- Determine the values of all the four quantum numbers of the 8th electr...
Text Solution
|
- The quantum mechanical treatment of the hydrogen atom gives the energy...
Text Solution
|
- How fast must a 54g tennis ball travel in order to have a de Broglie w...
Text Solution
|
- For the following, give the sub level designation, the allowable m val...
Text Solution
|
- An atom of an element contains 35 electrons and 45 neutrons. Deduce (...
Text Solution
|