A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-THE D-AND F-BLOCK ELEMENTS TEST-SINGLE CHOICE
- In which of the following Mn has highest oxidation state?
Text Solution
|
- Compound that is both paramagnetic and coloured is
Text Solution
|
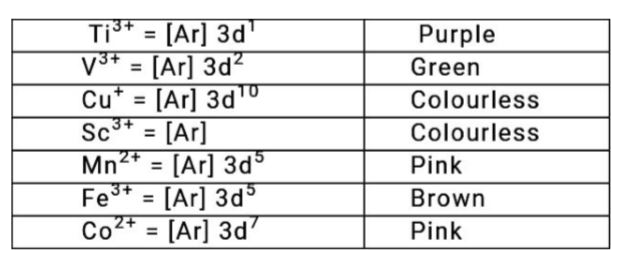
- Out of the following ions Ti^(3+), V^(3+), Cu^(+), Sc^(3+), Mn^(2+), F...
Text Solution
|
- The basic character of the transition metal monoxides follows the orde...
Text Solution
|
- Among the following ions, which one will have the highest paramagnetic...
Text Solution
|
- Amongst "TiF "(6)^(2-),"CoF "(6)^(3-), CuCl and NiCl(4)^(2-) (atomic ...
Text Solution
|
- Among the following transition elements, pick out the element(s) with ...
Text Solution
|
- The sulphate of a metal A on heating gives two gases B and C and an ox...
Text Solution
|
- A green coloured solution of a salt, changes its colour to light pink ...
Text Solution
|
- Which one of the following transition metal ions is diamagnetic?
Text Solution
|
- Which of the following pair of transition metal ions have the same cal...
Text Solution
|
- Which of the following is coloured compound?
Text Solution
|
- In context with the transition elements, which of the following statem...
Text Solution
|
- In the context of the lanthanides, which of the following statements i...
Text Solution
|
- The transition elements are more metallic than the representative elem...
Text Solution
|
- The Potassium manganate (K(2) MnO(4)) is formed, when
Text Solution
|
- The calculated value of magnetic moment of Fe^(3+) is
Text Solution
|
- The pair in which both species have same magnetic moment is -
Text Solution
|
- Cr^(2+) and Mn^(3+) both have d^(4) configuration. Thus
Text Solution
|
- Match the catalysts to the correct process.
Text Solution
|