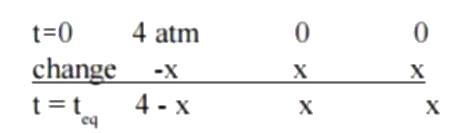A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-EQUILIBRIUM TEST (CHEMICAL)-1-SINGLE CHOICE
- The equilibrium, SO(2)Cl(2)(g) hArr SO(2) (g) + Cl(2) (g) is attained...
Text Solution
|
- The reaction quotient (Q) predicts:
Text Solution
|
- K(p) = 0.04 atm at 899 K for the equilibrium shown below. What is the ...
Text Solution
|
- The equilibrium constant K(c ) for A (g) hArr B (g) is 1.1, gas B will...
Text Solution
|
- X(2) + X^(-) hArr X(3)^(-) (x = iodine) This reaction is set up in aqu...
Text Solution
|
- The partial pressure of CH(3) OH (g), CO((g)) and H(2(g)) in equilibr...
Text Solution
|
- 0.2 mole of NH4Cl are introduced into an empty container of 10 litre a...
Text Solution
|
- For the reaction : CH(4(g)) +2O(2(g)) hArr CO(2(g)) +2H(2)O((l)), W...
Text Solution
|
- In which of the following reactions, increase in the volume at constan...
Text Solution
|
- For the reversible reaction, N(2)(g) +3H(2)(g) hArr 2NH(3)(g) at 50...
Text Solution
|
- At constant temperature, the equilibrium constant (K(p)) for the decom...
Text Solution
|
- In which of the following equilibrium, change in the volume of the sys...
Text Solution
|
- The equilibrium constant for the disproportionation of HgCl^(+) and Hg...
Text Solution
|
- Change in volume of the system does not alter the number of moles in w...
Text Solution
|
- For the following three reaction (i), (ii) and (iii) equilibrium const...
Text Solution
|
- The pK(a) of a weak acid, HA, is 4.80. The pK(b) of a weak base, BOH ...
Text Solution
|
- Which of the following buffer solutions turns invalid on addition of 1...
Text Solution
|
- The two equilibrium, AB hArr A^(+) +B^(-) and AB + B^(-)hArr AB(2)^(-)...
Text Solution
|
- An equilibrium mixture at 300 K has N(2)O(4) and NO(2) at 0.28 atm and...
Text Solution
|
- The Henderson's equation for acetic acid and sodium acetate buffer is ...
Text Solution
|