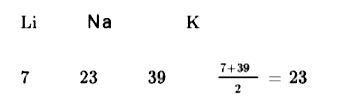A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES TEST -SINGLE CHOICE
- The correct order of increasing oxidising power is
Text Solution
|
- Which of the following is a typical element?
Text Solution
|
- Calculate the electronegativity of fluorine using the following data. ...
Text Solution
|
- Which of the following set of elements can not be a triad ?
Text Solution
|
- Which among the following is the incorrect order of size?
Text Solution
|
- The first ionisation energies in eV/atom of magnesium and aluminium ar...
Text Solution
|
- Which one of the following is not a representative element?
Text Solution
|
- The 15 elements have been placed in VI period and third group of the p...
Text Solution
|
- The atomic masses of Li and K are 7 and 39, respectively. According to...
Text Solution
|
- A trend which is common to elements of both the group 1st and group 17...
Text Solution
|
- Which among the following statements is correct?
Text Solution
|
- Which of the following is incorrect ?
Text Solution
|
- The ionic radii (in Å) of N^(3-),O^(2-) and F^- are respectively:
Text Solution
|
- The attractive force exerted by an atom on an electron pair shared wit...
Text Solution
|
- The correct order of covalent, van der Waals and crystal radii is
Text Solution
|
- The effective nuclear charge for an electron in ""7N^14 will be (usin...
Text Solution
|
- What is the atomic number of the element with symbol Uus?
Text Solution
|
- The atomic number of an element is 35. What is the total number of ele...
Text Solution
|
- Which of the elements, whose atomic number are given below, cannot be ...
Text Solution
|
- The atomic number of element Unq is
Text Solution
|