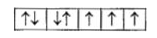
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The number of unpaired electrons in ""(27)Co is
Text Solution
|
- The number of unpaired electrons in the complex ion [CoF6]^(3-) is (At...
Text Solution
|
- The number of unpaired electrons in the complex ion [CoF6]^(3-) is (At...
Text Solution
|
- Co^(2+)(Z=27) में अयुग्मित इलेक्ट्रॉनों की संख्या होगी :
Text Solution
|
- [CoF(6)]^(---) संकुल आयन में अयुग्मित इलेक्ट्रॉनों की संख्या होगी (Co ...
Text Solution
|
- [CoF(6)]^(3-) संकुल आयन में अयुग्मित इलेक्ट्रॉनों की संख्या होगी (Co क...
Text Solution
|
- The number of unpaired electrons in Ni (CO)(4) is
Text Solution
|
- The number of unpaired electrons in cobalt atoms is (atomic number of ...
Text Solution
|
- The number of unpaired electrons in [Ni(CO)(4)]^(0) is
Text Solution
|