A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
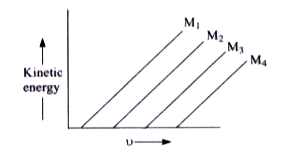
- A plot of the kinetic energy ((1)/(2) mv^(2)) of ejected electrons as...
Text Solution
|
- The lines y=m1x ,y=m2xa n dy=m3x make equal intercepts on the line x+...
Text Solution
|
- If (mi,1/mi),i=1,2,3,4 are concyclic points then the value of m1m2m3m4...
Text Solution
|
- If (mi,1/mi), i=1,2,3,4 are concyclic points, then the value of m1m2m3...
Text Solution
|
- A pot of the kinetic energy (1//2mv^(2)) of ejected electrons as a fun...
Text Solution
|
- A plot of the kinetic energy ((1)/(2)mv^(2)) of ejected electrons as a...
Text Solution
|
- सूत्र ((m(1)x(2)+m(2)x(1))/(m(1)+m(2)) , (m(1)y(2)+m(2)y(1))/(m(1) + ...
Text Solution
|
- Two masses m(1) and m(2) ( m(2) gt m(1)) are hanging vertically over f...
Text Solution
|
- If (m(1),1//m(1)), i=1,2,3,4 are concyclic points, then the value of m...
Text Solution
|