Text Solution
Verified by Experts
Topper's Solved these Questions
METALS AND NON-METALS
NAND LAL PUBLICATION|Exercise ADDITIONAL QUESTIONS|4 VideosMETALS AND NON-METALS
NAND LAL PUBLICATION|Exercise ACTIVITY 3.1|2 VideosMETALS AND NON-METALS
NAND LAL PUBLICATION|Exercise ACTIVITY 3.8|3 VideosCHEMICAL REACTIONS AND EQUATIONS
NAND LAL PUBLICATION|Exercise ADDITIONAL QUESTIONS|5 Videos
Similar Questions
Explore conceptually related problems
NAND LAL PUBLICATION-METALS AND NON-METALS-EXERCISES
- An element reacts with oxygen to give a compound with a high melting p...
Text Solution
|
- Food cans are coated with tin and not with zinc because:
Text Solution
|
- You are given a hammer, a battery, a bulb ,wires and a switch How coul...
Text Solution
|
- You are given a hammer, a battery, a bulb ,wires and a switch How coul...
Text Solution
|
- What are amphoteric oxides? give two examples of amphoteric oxides?
Text Solution
|
- Name two metals which will displace hydrogen from dilute acids, and tw...
Text Solution
|
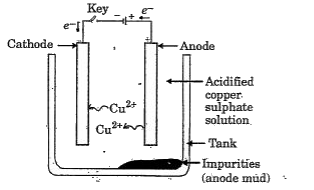
- In the electrolytic refining a metal M what would you take as the anod...
Text Solution
|
- Pratyush took sulphur powder on a spatula and heated it. He collecte...
Text Solution
|
- Pratyush took sulphur powder on a spatula and heated it. He collected ...
Text Solution
|
- Pratyush took sulphur powder on a spatula and heated it. He collected ...
Text Solution
|
- State two ways to prevent the rusting of iron
Text Solution
|
- What type of oxides are formed when non-metals combine with oxygen?
Text Solution
|
- Give reasons:Platinum,gold and silver are used to make jewellery
Text Solution
|
- Give reasons: sodium,potassium and lithium are stored under oil.
Text Solution
|
- Give reasons: aluminium is highly reactive metal, yet it is used to ma...
Text Solution
|
- Give reasons: Carbonate and suphide ores are usually converted into ox...
Text Solution
|
- You must have seen trainshed copper vessels being cleaned with lemon o...
Text Solution
|
- Differentiate between metals and non-metals on the basis of their chem...
Text Solution
|
- A man went door to door posing as a goldmith he promised to bring back...
Text Solution
|
- Give the reason why copper is used to make hot water tanks but steel (...
Text Solution
|