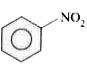
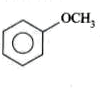
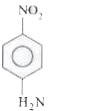
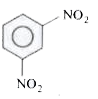
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In which of the following molecules, pi- electron density in ring is m...
Text Solution
|
- The correct order of electron density in aromatic ring of following co...
Text Solution
|
- The decreasing order of electron density on the ring is:
Text Solution
|
- Which molecules I showing pi -electrons alternate to pi -electron conj...
Text Solution
|
- Which of the following aromatic rings have greater electron density th...
Text Solution
|
- Which of the following aromatic ring have greater electron density tha...
Text Solution
|
- The correct decreasing order of electron density in aromatic ring of f...
Text Solution
|
- The decreasing order of electron density on the ring is :
Text Solution
|
- In this molecules, pi -electron density is more on :
Text Solution
|