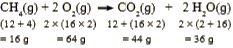Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Calculate the mass of carbon dioxide and water formed on complete comb...
Text Solution
|
- Calculate the formula mass of potassium carbonate (K(2)CO(3)) (Given :...
Text Solution
|
- Convert 22 g of carbon dioxide (CO(2)) into moles. (Atomic masses : C ...
Text Solution
|
- What is the mass of 0.5 mole of wate (H(2)O) . (Atomic masses : H = 1 ...
Text Solution
|
- Which contains more molecules, 4 g of methane (CH(4)) or 4g of oxyen (...
Text Solution
|
- If 1 g of sulphur dioxide contains x molecules, what will be the numb...
Text Solution
|
- The molecular formula of glucose is C(6)H(12)O(6). Calculate its molec...
Text Solution
|
- Calculate the molecular mass of ethanoic acid, CH(3)COOH. (Atomic ma...
Text Solution
|
- Calculate the molecular mass of nitric acid, HNO(3). (Atomic masses : ...
Text Solution
|