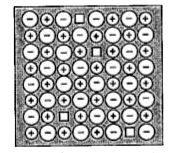
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Recommended Questions
- In these questions, a statement of Assertion followed by a statement o...
Text Solution
|
- NaCl shows Schottky defects and AgCl Frenkel defects. Their electrical...
Text Solution
|
- Out of NaCl and AgCl, which one shows Frenkel defect and why ?
Text Solution
|
- Assertion : Due to Frenkel defect, density of the crystalline solid de...
Text Solution
|
- In the following questions a statement of Assertin (A) followed by a s...
Text Solution
|
- In the following questions a statement of Assertin (A) followed by a s...
Text Solution
|
- Assert : due to Frenkel defect, density of the crystalline solid decra...
Text Solution
|
- Give reasons : In stoichiometric defects, NaCl exhibits Schottky defec...
Text Solution
|
- Assertion : due to Frenkel defect, density of the crystalline solid de...
Text Solution
|