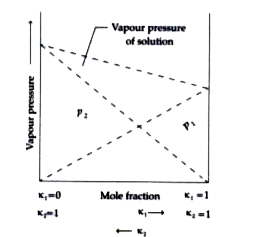
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Read the passage given below and answer the following questions : Ra...
Text Solution
|
- For a solution of volatile liquids the partial vapour pressure of each...
Text Solution
|
- state Dalton's law of partial pressure.
Text Solution
|
- State Dalton's law of Partial Pressure.
Text Solution
|
- State Dalton's law of partial pressure,
Text Solution
|
- किसी विलयन घटक का आंशिक दाब इसके मोल-अंश के समानुपाती होता है। इसे कहा...
Text Solution
|
- State Dalton's law of partial pressure.
Text Solution
|
- State Dalton's law of Partial pressures.
Text Solution
|
- Partial pressure of a solution component is directly proportional to i...
Text Solution
|