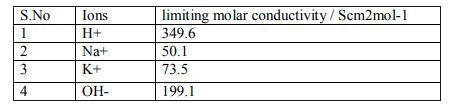
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following option will be the limiting molar conductivity ...
Text Solution
|
- Limiting molar conductivity of CH(3)COOH (i.e. ^^(m(CH(3)COOH))^(0) is...
Text Solution
|
- The molar conductance of HCl, NaCl and CH(3)COONa are 426,12 6 and 91 ...
Text Solution
|
- The molar conductance of NaCl, HCl and CH(3)COONa at infinite dilution...
Text Solution
|
- The molar conductance of NaCl, HCl and CH(3)COONa at infinite dilution...
Text Solution
|
- Limiting molar conductivity of NaBr is
Text Solution
|
- What is limiting molar conductivity?
Text Solution
|
- Limiting molar conductivity of Mg^(2+) and Cl^(-) ions in water is 10...
Text Solution
|
- Define limiting molar conductivity?
Text Solution
|