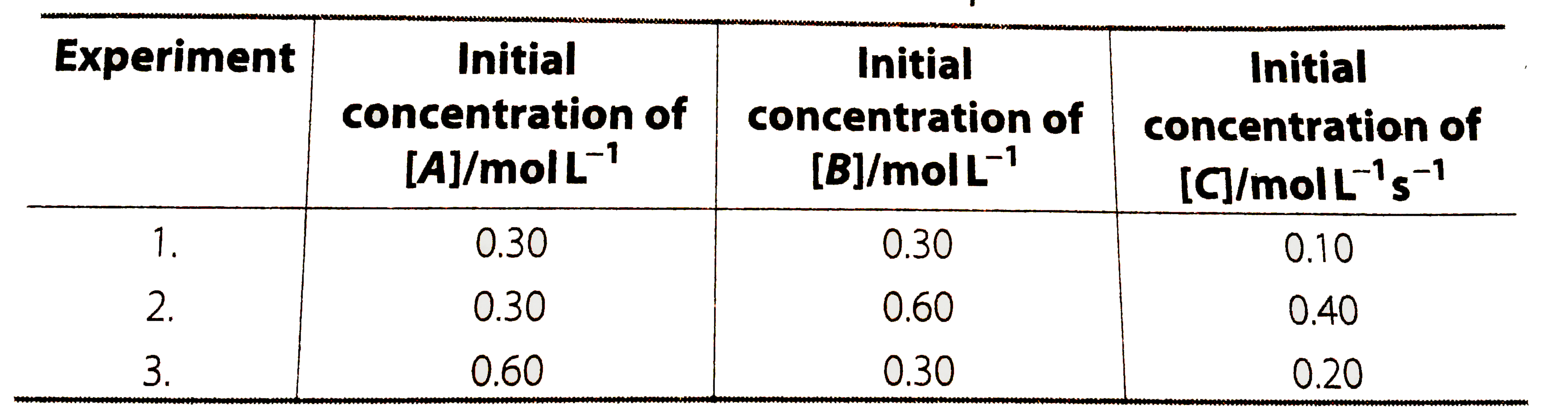
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Recommended Questions
- Compounds 'A' and 'B' react according to the following chemical eq...
Text Solution
|
- Compounds A and B react according to the following chemical equation :...
Text Solution
|
- Compounds A and B react according to the following chemical equation :...
Text Solution
|
- Compounds 'A' and 'B' react according to the following chemical equati...
Text Solution
|
- Compounds 'A' and 'B' react according to the following chemical eq...
Text Solution
|
- Compound 'A' and B react according to the following chemical equation....
Text Solution
|
- गैसीय अभिक्रिया A + 2B hArr 2C + D में B का प्रारंभिक सांद्रण A का 1.5...
Text Solution
|
- A reaction, A(g)+2B(g)hArr 2C(g)+D(g) was studied using an initial con...
Text Solution
|
- एक रासायनिक अभिक्रिया , A+2B overset(K) hArr 2C +D में, B की प्रारम्भि...
Text Solution
|