A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Recommended Questions
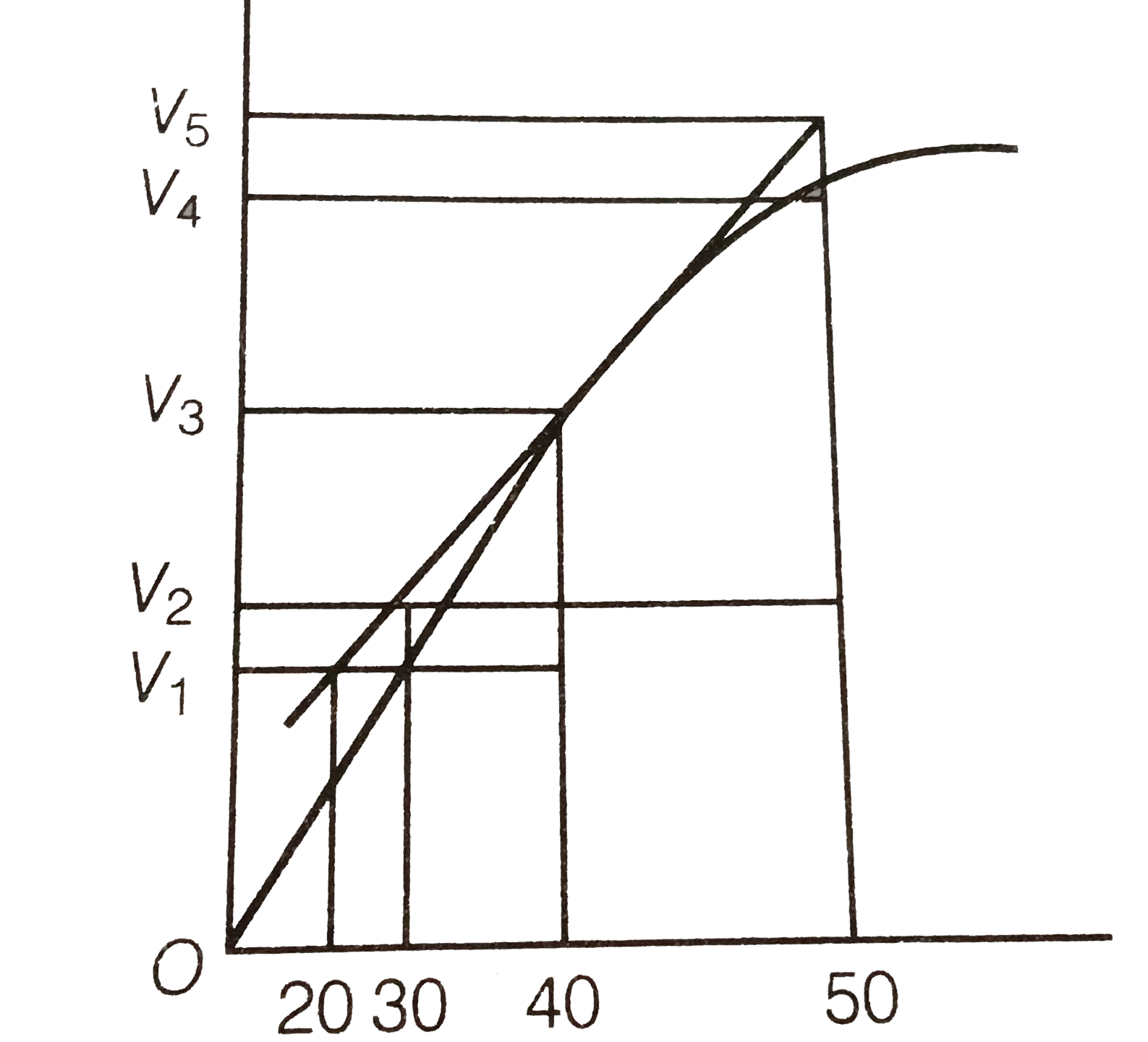
- A graph of volume of hydrogen released vs time for the reaction b...
Text Solution
|
- Density vs volume graph is shown in the figure. Find corresponding pre...
Text Solution
|
- For a first order reaction: A(3) rarr 3A, Following graph is observed ...
Text Solution
|
- A graph of volume of hydrogen released us. Time for the reaction betwe...
Text Solution
|
- A graph of volume of hydrogen released vs time for the reaction b...
Text Solution
|
- A graph of volume of hydrogen released vs time for the reaction betwee...
Text Solution
|
- A graph of volume of hydrogen released vs time for the reaction betwee...
Text Solution
|
- A graph of volume of hydrogen released vs time for the reaction b...
Text Solution
|
- The graph between the volume and time of hydrogen formed in the reacti...
Text Solution
|