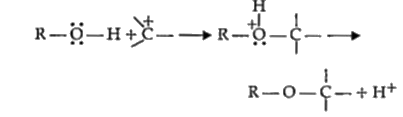A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Recommended Questions
- I. Read the passage given below and answer the following questions : ...
Text Solution
|
- Which of the following can act both as an electrophile and a nucleophi...
Text Solution
|
- H(2)C = O or CH(3)CN acts as a nucleophile as well as an electrophile....
Text Solution
|
- Assertion : Alcohols react both as nucleophiles and electrophiles. Rea...
Text Solution
|
- Why the compound CH(3) CN can act both as nucleophile and electrophile...
Text Solution
|
- The bond angle between C-O and O-H bonds in alcohols is close to
Text Solution
|
- A : In esterification reaction alcohol act as nucleophile . R : In thi...
Text Solution
|
- Ethyl alcohol acts as nucleophile when it reacts with
Text Solution
|
- Assertion : Alcohols react both as nucleophiles and electrophiles. ...
Text Solution
|