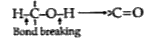A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- II. Read the passage given below and answer the following questions : ...
Text Solution
|
- Organic reactions take place through the formations of reactive carbon...
Text Solution
|
- Which of the following reactions of an alcohol does not involve O-H bo...
Text Solution
|
- In which of the following reactions of alcohol there is no cleavage of...
Text Solution
|
- In which of the following reactions of alcohol there is no cleavage of...
Text Solution
|
- Give one reaction of alcohol involving cleavage of : (i) C – O bond (i...
Text Solution
|
- The heterolytic cleavage of C-H bonds results in the formation of
Text Solution
|
- The cleavage of C-H bond in aldehydes leads to formation of .............
Text Solution
|
- What is the reactivity order of primary secondary and tertiary alcohol...
Text Solution
|