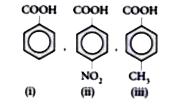
A
B
C
D
Text Solution
Verified by Experts
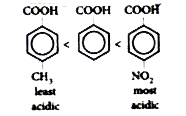
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Arrange the following compounds in increasing order of acid strength
Text Solution
|
- Arrange in the order of increasing acidic strengths.
Text Solution
|
- Arrange the following compounds in decreasing order of their acidic st...
Text Solution
|
- Arrange the following compounds in order of decreasing acidic strength...
Text Solution
|
- Arrange the following compounds in order of decreasing acidic strength...
Text Solution
|
- Arrange the following compounds in increasing order of their acidic st...
Text Solution
|
- Arrange in order of increasing acidic strength
Text Solution
|
- Arrange the following compounds in order of decreasing acidic strength...
Text Solution
|
- Consider the compound and arrange X,Y, Z in order of increasing acid s...
Text Solution
|