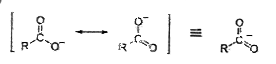A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Recommended Questions
- Assertion: Carboxylic acids are more acidic than phenols. Reason: Ph...
Text Solution
|
- Carboxylic acids are more acidic than phenol and alcohol because of
Text Solution
|
- समझाइए की क्यों ऑर्थोनाइट्रो फीनॉल, ऑर्थो मेथॉक्सी फीनॉल से अधिक अम्ल...
Text Solution
|
- Assertion : Ortho nitro phenol is more acidic than meta nitro phenol ...
Text Solution
|
- कार्बोक्सिलक अम्ल्, फीनॉल की अपेक्षा अधिक अम्लीय होता है। समझाइए।
Text Solution
|
- समझाइये क्यों आर्थों-नाइट्रोफिनॉल आर्थों-मिथाक्सी-फिनॉल से ज्यादा अम्ल...
Text Solution
|
- How many of these compounds are more acidic than phenol here. Formic a...
Text Solution
|
- Carboxylic acids are more acidic than phenols. Give reason.
Text Solution
|
- Assertion: Carboxylic acids are more acidic than phenols. Reason: Ph...
Text Solution
|