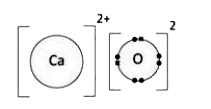
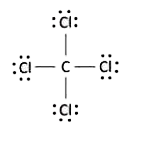
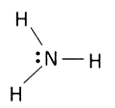
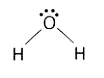
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- "" is NOT a covalent molecule
Text Solution
|
- आप किस आधार पर यह कह सकते हैं की स्केंडियम (Sc = 21) एक संक्रमण तत्व ह...
Text Solution
|
- जीनॉन का कौन - सा फ़्लुओराइड संभव नहीं है ?
Text Solution
|
- SO(2) अणु में सल्फर परमाणु का संकरण है -
Text Solution
|
- निम्नलिखित में से कोन-सा ग्राफ द्विघात बहुपद का नहीं है ?
Text Solution
|
- दिष्ट धरा परिपथ में ट्रांसफॉर्मर का उपयोग क्यों नहीं किया जाता है ?
Text Solution
|
- किसी ट्रांसफॉर्मर में क्या सम्भव नहीं हैं ?
Text Solution
|
- किसी ऐसे वर्ष में जो लीप वर्ष न हो 53 रविवार होने की प्रायिकता क्या है...
Text Solution
|
- निम्न में से कौन-सा ठोस अवस्था में सहसंयोजक क्रिस्टल है ?
Text Solution
|