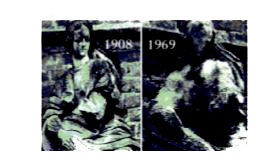
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL REACTIONS AND EQUATIONS
OSWAL PUBLICATION|Exercise ASSERTION AND REASON BASED MCQs |8 VideosCHEMICAL REACTIONS
OSWAL PUBLICATION|Exercise BOARD CORNER (Short Answer Type Questions) |3 VideosDIKSHA QUESTIONS
OSWAL PUBLICATION|Exercise PERIODIC CLASSIFICATION OF ELEMENTS (MULTIPLE CHOICE QUESTIONS) |13 Videos
Similar Questions
Explore conceptually related problems
OSWAL PUBLICATION-CHEMICAL REACTIONS AND EQUATIONS-CASE-BASED MCQs
- Marble’s popularity began in ancient Rome and Greece, where white and ...
Text Solution
|
- Read the following and answer the question. Marble's popularity be...
Text Solution
|
- Marble’s popularity began in ancient Rome and Greece, where white and ...
Text Solution
|
- Marble’s popularity began in ancient Rome and Greece, where white and ...
Text Solution
|
- Read the following and answer the question: Chemistry in Automobiles...
Text Solution
|
- Read the following and answer the question: Chemistry in Automobiles...
Text Solution
|
- For an internal combustion engine to move a vehicle down the road ,it ...
Text Solution
|
- For an internal combustion engine to move a vehicle down the road,it m...
Text Solution
|
- For an internal combustion engine to move a vehicle down the road, it ...
Text Solution
|
- Read the given passage and answer the questions. The physical state...
Text Solution
|
- Read the given passage and answer the questions. The physical state...
Text Solution
|
- Read the given passage and answer the questions. The physical state...
Text Solution
|
- Read the given passage and answer the questions. The physical state...
Text Solution
|
- Read the given passage and answer the questions. The physical state...
Text Solution
|
- Read the given passage and answer the questions. In the following c...
Text Solution
|
- Read the given passage and answer the questions. In the following c...
Text Solution
|
- Read the given passage and answer the questions. In the following c...
Text Solution
|
- Read the given passage and answer the questions. In the following c...
Text Solution
|
- Read the given passage and answer the questions. P, Q and R are thr...
Text Solution
|
- Read the given passage and answer the questions. P, Q and R are thr...
Text Solution
|