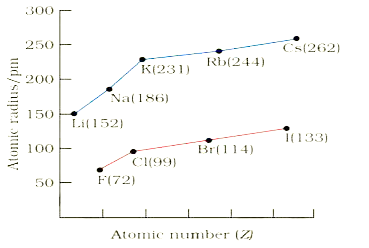
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
OSWAL PUBLICATION-METALS AND NON - METALS -CASE - BASED MCQs
- Read the following and answer any four questions When a silvery grey...
Text Solution
|
- Read the following and answer any four questions Sohan went door to ...
Text Solution
|
- Read the following and answer any four questions Sohan went door to ...
Text Solution
|
- Read the following and answer any four questions Sohan went door to ...
Text Solution
|
- Read the following and answer any four questions Sohan went door to ...
Text Solution
|
- Read the following and answer any four questions Sohan went door to ...
Text Solution
|
- Read the following and answer any four questions During extraction o...
Text Solution
|
- Read the following and answer any four questions During extraction o...
Text Solution
|
- Read the following and answer any four questions During extraction o...
Text Solution
|
- Read the following and answer any four questions During extraction o...
Text Solution
|
- Read the following and answer any four questions During extraction o...
Text Solution
|
- Read the following and answer the question Metallic Character: The...
Text Solution
|
- Metallic Character The ability of an atom to donate electrons and fo...
Text Solution
|
- Metallic Character The ability of an atom to donate electrons and fo...
Text Solution
|
- Metallic Character The ability of an atom to donate electrons and fo...
Text Solution
|
- Metallic Character The ability of an atom to donate electrons and fo...
Text Solution
|
- In a thermite reaction, a compound of iron reacts with a metal. The ...
Text Solution
|
- In a thermite reaction, a compound of iron reacts with a metal. Afte...
Text Solution
|
- In a thermite reaction, a compound of iron reacts with a metal. The ...
Text Solution
|
- In a thermite reaction, a compound of iron reacts with a metal. The ...
Text Solution
|