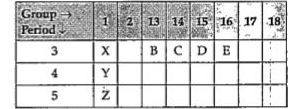
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS
OSWAL PUBLICATION|Exercise SELF - ASSESSMENT - 1 ( OBJECTIVE TYPE QUESTIONS) |13 VideosPERIODIC CLASSIFICATION OF ELEMENTS
OSWAL PUBLICATION|Exercise SELF - ASSESSMENT - 2 ( OBJECTIVE TYPE QUESTIONS) |15 VideosPERIODIC CLASSIFICATION OF ELEMENTS
OSWAL PUBLICATION|Exercise ASSERTION AND REASON BASED MCQs|9 VideosOLYMPIAD 2019 - 20
OSWAL PUBLICATION|Exercise CHEMISTRY QUESTIONS (Is Matter Around us Pure)|1 VideosSAMPLE PAPER 1
OSWAL PUBLICATION|Exercise QUESTION BANK|9 Videos
Similar Questions
Explore conceptually related problems
OSWAL PUBLICATION-PERIODIC CLASSIFICATION OF ELEMENTS -CASE - BASED MCQs
- Mendeleev was a Russian chemist, who contributed the most for the deve...
Text Solution
|
- Mendeleev was a Russian chemist, who contributed the most for the deve...
Text Solution
|
- Mendeleev was a Russian chemist, who contributed the most for the deve...
Text Solution
|
- Mendeleev was a Russian chemist, who contributed the most for the deve...
Text Solution
|
- Mendeleev was a Russian chemist, who contributed the most for the deve...
Text Solution
|
- Which of these elements have smallest atomic size?
Text Solution
|
- Write valency of element E.
Text Solution
|
- Identify the elements which have similar chemical properties as the el...
Text Solution
|
- The number of period that the modern periodic table has
Text Solution
|
- Which of them will have largest atomic radii:
Text Solution
|
- Atoms of eight elements A, B, C, D, E, F, G and H have the same number...
Text Solution
|
- Atoms of eight elements A, B, C, D, E, F, G and H have the same number...
Text Solution
|
- Atoms of eight elements A, B, C, D, E, F, G and H have the same number...
Text Solution
|
- Atoms of eight elements A, B, C, D, E, F, G and H have the same number...
Text Solution
|
- Atoms of eight elements A, B, C, D, E, F, G and H have the same number...
Text Solution
|
- Which is the most reactive metal ?
Text Solution
|
- Name the family of fluorine Q, R, T:
Text Solution
|
- Which of the following element belongs to group 2?
Text Solution
|
- Which other element is likely to present in the group in which fluori...
Text Solution
|
- Name the element P placed below Carbon in group 14:
Text Solution
|