A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-ORGANIC COMPOUNDS CONTAINING OXYGEN ATOM -OLYMPIAD AND NTSE LEVEL EXERCISES
- Primary and secondary alcohols on action of reduced copper give
Text Solution
|
- Ethyl alcohol on oxidation with K(2)Cr(2)O(7) gives
Text Solution
|
- In the following series of chemical reactions, identify Z C(3)H(7)OH...
Text Solution
|
- When glycerol is heated with KHSO(4) it gives
Text Solution
|
- The enol form of acetone, after treatment with D(2)O, gives
Text Solution
|
- Among the given compounds, the most susceptible to nucleophilic attack...
Text Solution
|
- Acetic acid reacts with PCl(5) to form
Text Solution
|
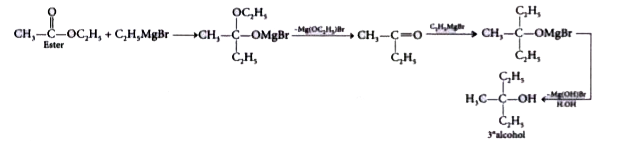
- CH(3)COOC(2)H(5) with excess of C(2)H(5)MgBr and hydrolysis gives
Text Solution
|
- the most reactive compound towards formation of cyanohydrin on treatme...
Text Solution
|
- The synthesis of crotonaldehyde from acetaldehyde is an example of ….....
Text Solution
|