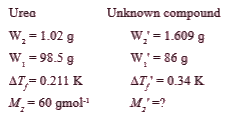Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- 1.02 g of urea when dissolved in 98.5 g of certain solvent decreases i...
Text Solution
|
- Calculatate the mass of compound (molar mass = 256 g mol^(-1) be the d...
Text Solution
|
- When 0.01 mole of sugar is dissolved in 100g of a solvent, the glucose...
Text Solution
|
- The molarity of urea (molar mass 60 g mol^(-1) ) solution by dissolvin...
Text Solution
|
- If 1 g of solute (molar mass = 50 g mol^(-1) ) is dissolved in 50 g of...
Text Solution
|
- The molarity of urea (molar mass 60 g mol^(-1) ) solution by dissolvin...
Text Solution
|
- The molarity of urea (molar mass 60 g mol^(-1)) solution by dissolving...
Text Solution
|
- Pure solvent A has freezing point 16.5^(@)C . On dissolving 0.4 g of B...
Text Solution
|
- Calculate the mass of compound (molar mass = "256 g mol"^(-1) ) to be ...
Text Solution
|