Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
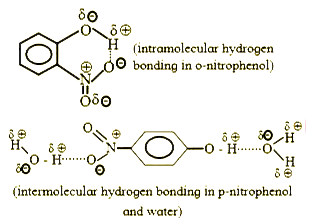
- The solubility of o-nitrophenol and p-nitrophenol is 0.2 g and 1.7 g /...
Text Solution
|
- Out of o-nitrophenol and p-nitrophenol, which is more volatile ? Expla...
Text Solution
|
- (A) A mixture of o-nitrophenol and p-nitrophenol can be separated by s...
Text Solution
|
- Out of o-nitrophenol annd p-nitrophenol, which is more volatile? Expla...
Text Solution
|
- o Nitrophenol m and p are less soluble in water than nitrophenol.
Text Solution
|
- The solubility of o-nitrophenol in water is lower than that of p- and ...
Text Solution
|
- Out of o - nitrophenol and p - nitrophenol, which is more volatile? Ex...
Text Solution
|
- o-नाइट्रोफ़ीनॉल और p-नाइट्रोफ़ीनॉल में से कौन-सा अधिक वाष्पशील है? स्प...
Text Solution
|
- Which explains that O- nitrophenol is more volatile than p- nitropheno...
Text Solution
|