When you spin the wheel shown, What are the possible outcomes?
(Out comes here means the possible sector where the pointer stops)
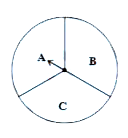
When you spin the wheel shown, What are the possible outcomes?
(Out comes here means the possible sector where the pointer stops)

(Out comes here means the possible sector where the pointer stops)

Similar Questions
Explore conceptually related problems
When you roll a die, What are the six possible outcomes?
If you try to start a scooter , What are the possible outcomes?
A die has six faces numbered from 1 to 6. It is rolled and the number on the top face is noted. When this is treated as a random trial. a) What are the possible outcomes ? b)Are they equally likely? Why? c) Find the probability of a composite number turning up on the top face.
A spinner has four colours as shown in the figure. When we spin it once, find a) At which colour, is the pointer more likely to stop? b) At which colour, is the pointer less likely to stop? c) At which colours, is the pointer equally likely to stop? d) What is the chance the pointer will stop on white? e) Is there any colour at which the pointer certainly stops?
When die is rolled once unbiased what is the probability of getting a multiple of 3 out of possible outcomes?
Think of this puzzle What do you need to find a chosen number from this square? Four of the clues below are true but do nothing to help in finding the number. Four of the clues are necessary for finding it. Here are eight clues to use: a. The number is greater than 9. b. The number is not a multiple of 10. c. The number is a multiple of 7. d. The number is odd. e. The number is not a multiple of 11. f. The number is less than 200. g. Its ones digit is larger than its tens digit. h. Its tens digit is odd. What is the number? Can you sort out the four clues that help and the four clues that do not help in finding it? First follow the clues and strike off the number which comes out from it. Like - from the first clue we come to know that the number is not from 1 to 9. (strike off numbers from 1 to 9). After completing the puzzle, see which clue is important and which is not?
Answer carefully: (a) Two large conducting spheres carrying charges Q_(1) and Q_(2) are brought close to each other. Is the magnitude of electrostatic force between them exactly given by Q_(1),Q_(2)//4pi epsilon_(0)r^(2) , where r is the distance between their centres? (b) If Coulomb’s law involved 1//r^(3) dependence (instead of would Gauss’s law be still true ? (c) A small test charge is released at rest at a point in an electrostatic field configuration. Will it travel along the field line passing through that point? (d) What is the work done by the field of a nucleus in a complete circular orbit of the electron? What if the orbit is elliptical? (e) We know that electric field is discontinuous across the surface of a charged conductor. Is electric potential also discontinuous there? (f) What meaning would you give to the capacitance of a single conductor? (g) Guess a possible reason why water has a much greater dielectric constant (= 80) than say, mica (= 6).
On the basis of the postulates of kinetic theory of gases, it is possible to derive the mathematical expression, commonly known as kinetic gas equation. PV = 1/3 m n u^3? where, P= Pressure of the gas, V a volume of the gas, m=Mass of a molecule, n = Number of molecules present in the given amount of a gas and u = root mean square speed For one mole of gas, PV = RT and n=N_A 1/3 m N_a u^2 = RT or 2/3 .1/2m N_A u^2 = N_A [1/2mN_Au^2 = KE "per mole"] ,2/3K.E. = RT implies K.E. 3/2RT Average kinetic energy per mol does not depend on the nature of the gas but depends only on temperature. This, when two gases are mixed at the same temperature, there will be no rise or decrease in temperature unless both react chemically. Average kinetic energy per molecule = ("Average K.E. per mole")/N = 3/2(RT)/(N) implies 3/2kT where k is the Boltzmann constant In deriving the kinetic gas equation, the use of the root mean square speed of the molecules is done, hecause it is
On the basis of the postulates of kinetic theory of gases, it is possible to derive the mathematical expression, commonly known as kinetic gas equation. PV = 1/3 m n u^3? where, P= Pressure of the gas, V a volume of the gas, m=Mass of a molecule, n = Number of molecules present in the given amount of a gas and u = root mean square speed For one mole of gas, PV = RT and n=N_A 1/3 m N_a u^2 = RT or 2/3 .1/2m N_A u^2 = N_A [1/2mN_Au^2 = KE "per mole"] ,2/3K.E. = RT implies K.E. 3/2RT Average kinetic energy per mol does not depend on the nature of the gas but depends only on temperature. This, when two gases are mixed at the same temperature, there will be no rise or decrease in temperature unless both react chemically. Average kinetic energy per molecule = ("Average K.E. per mole")/N = 3/2(RT)/(N) implies 3/2kT where k is the Boltzmann constant The average kinetic energy (in joule) of the molecules in 8g methane at 27&@C is.
On the basis of the postulates of kinetic theory of gases, it is possible to derive the mathematical expression, commonly known as kinetic gas equation. PV = 1/3 m n u^3? where, P= Pressure of the gas, V a volume of the gas, m=Mass of a molecule, n = Number of molecules present in the given amount of a gas and u = root mean square speed For one mole of gas, PV = RT and n=N_A 1/3 m N_a u^2 = RT or 2/3 .1/2m N_A u^2 = N_A [1/2mN_Au^2 = KE "per mole"] ,2/3K.E. = RT implies K.E. 3/2RT Average kinetic energy per mol does not depend on the nature of the gas but depends only on temperature. This, when two gases are mixed at the same temperature, there will be no rise or decrease in temperature unless both react chemically. Average kinetic energy per molecule = ("Average K.E. per mole")/N = 3/2(RT)/(N) implies 3/2kT where k is the Boltzmann constant Which of the following expressions correctly represents the relationship between the average molar kinetic energies of CO and N_2 molecules at the same temperature ?
Recommended Questions
- When you spin the wheel shown, What are the possible outcomes? (Out ...
Text Solution
|
- When you spin the wheel shown,what are the possible outcomes.(see figu...
Text Solution
|
- Determine the point of symmetry of a regular hexagon. <img src="htt...
Text Solution
|
- Match the following Column A to Column B
Text Solution
|
- Match the following Column A to Column B
Text Solution
|
- Match the following Column A to Column B
Text Solution
|
- The inequation represented by the graph given below is : <img src="htt...
Text Solution
|
- The inequation that best describes the graph given below is <img src=...
Text Solution
|
- The inequation that best describes the following graph is <img src="h...
Text Solution
|