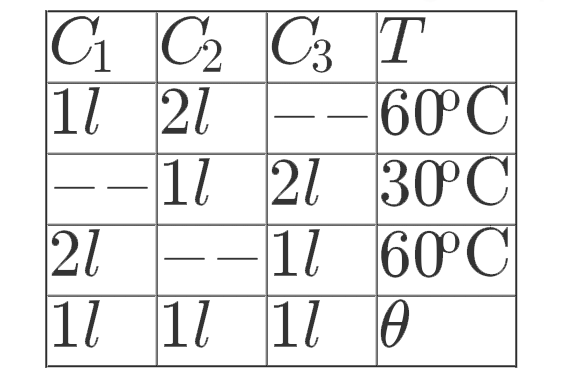A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
EDUCART PUBLICATION-SAMPLE PAPER - 11-SECTION-C
- The lower part of the metallic container is right circular cylinder an...
Text Solution
|
- Google maps cartography team is working on improving the scalability q...
Text Solution
|
- In the figure ,find the value of x.
Text Solution
|
- This bar graph shows the temperatures in degree Celsius in different c...
Text Solution
|
- The lower part of the metallic container is right circular cylinder an...
Text Solution
|
- Three containers C(1), C(2) and C(3) have water at different temperatu...
Text Solution
|
- In /\ABC, right angled at B, AB=3, BC=4 and CA=5. Then value of tan^2C...
Text Solution
|
- In /\ABC, right angled at B, AB=3, BC=4 and CA=5. Then value of sec^2A...
Text Solution
|
- In /\ABC, right angled at B, AB=3, BC=4 and CA=5. Then value of sin^2A...
Text Solution
|
- In /\ABC, right angled at B, AB=3, BC=4 and CA=5. Then value of cot^2C...
Text Solution
|