A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
JEE ADVANCED PREVIOUS YEAR-JEE ADVANCED 2021-QUESTION
- Match the following columns
Text Solution
|
- Among the following conformation that corresponds to the most stable c...
Text Solution
|
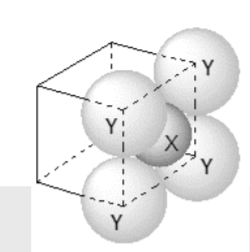
- For the given closed packed structure of a salt made of cation X and a...
Text Solution
|
- The calculated spin only magnetic moments of [Cr(NH3)6]^(3+) and [CuF6...
Text Solution
|
- For the following reaction scheme, percentage yields are given along t...
Text Solution
|
- For the following reaction scheme, percentage yields are given along t...
Text Solution
|
- For the reaction , X(s) harr Y(s) + Z(g), the plot of ln(pz)/p^(theta)...
Text Solution
|
- For the reaction , X(s) harr Y(s) + Z(g), the plot of ln(pz)/p^(theta)...
Text Solution
|
- The boilingpoint of water in a 0.1 molal silver nitrate solution(solut...
Text Solution
|
- The boilingpoint of water in a 0.1 molal silver nitrate solution(solut...
Text Solution
|
- The compound, which on reaction with HNO3 will give the product having...
Text Solution
|
- The reaction of Q with PhSNa yields an organic compound(major product)...
Text Solution
|
- The correct statement(s) related to colloids is(are)
Text Solution
|
- An ideal gas undergoes a reversible isothermal expansion from state I ...
Text Solution
|
- The correct statement(s) related to the metal extraction processes is(...
Text Solution
|
- A mixture of two salts is used to prepare a solution S, which gives th...
Text Solution
|
- The maximum number of possible isomers(including stereoisomers) which ...
Text Solution
|
- In the reaction given below, the total number of atoms having sp^2 hyb...
Text Solution
|
- The total number of possible isomers for [Pt(NH3)4Cl2]Br2 is ---------...
Text Solution
|
- The reaction sequence(s) that would lead to o-xylene as the major prod...
Text Solution
|