A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
JEE ADVANCED PREVIOUS YEAR-JEE ADVANCED 2021-QUESTION
- The total number of possible isomers for [Pt(NH3)4Cl2]Br2 is ---------...
Text Solution
|
- The reaction sequence(s) that would lead to o-xylene as the major prod...
Text Solution
|
- Correct option(s) for the following sequence of reactions is(are)
Text Solution
|
- For the following reaction 2X+Y overset(K)rarrP the rate of reacti...
Text Solution
|
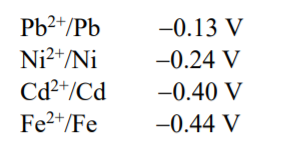
- Some standard electrode potentials at 298K are given below: To a ...
Text Solution
|
- The pair(s) of complexes where in both exhibit tetrahedral geometry is...
Text Solution
|
- The correct statement(s) related to oxoacids of phosphorous is(are)
Text Solution
|
- At 298K, the limiting molar conductivity of a weak monobasic acid is 4...
Text Solution
|
- At 298K, the limiting molar conductivity of a weak monobasic acid is 4...
Text Solution
|
- Reaction of xg of Sn with HCl quantitatively produced a salt. Entire a...
Text Solution
|
- Reaction of xg of Sn with HCl quantitatively produced a salt. Entire a...
Text Solution
|
- A sample (5.6g) containing iron is completely dissolved in cold dilute...
Text Solution
|
- A sample (5.6g) containing iron is completely dissolved in cold dilute...
Text Solution
|
- The amount of energy required to break a bond is same as the amount of...
Text Solution
|
- The amount of energy required to break a bond is same as the amount of...
Text Solution
|
- The reaction of K3[Fe(CN)6] eith freshly prepared FeSO4 solution procu...
Text Solution
|
- The reaction of K3[Fe(CN)6] eith freshly prepared FeSO4 solution procu...
Text Solution
|
- 1 mole of an ideal gas at 900K, undergoes 1 reversible processes, I fo...
Text Solution
|
- Consider a helium(He) atom that absorbs a photon of wavelength 330nm. ...
Text Solution
|
- Ozonolysis of ClO2 produces an oxide of chlorine. The average oxidatio...
Text Solution
|