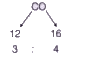
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The ratio in weight by which carbon and oxygen combine in a molecule ...
Text Solution
|
- In a carbon monoxide molecule, the carbon and the oxygen atoms are sep...
Text Solution
|
- Carbon and oxygen combine to form two oxides, carbon monoxide and carb...
Text Solution
|
- भार में वह अनुपात, जिसके द्वारा कार्बन तथा ऑक्सीजन संयोग करके कार्बन म...
Text Solution
|
- Assertion : Carbon monoxide is a poisonous gas Reason : Carbon monox...
Text Solution
|
- कार्बन डाइऑक्साइड के अणु में कार्बन तथा ऑक्सीजन का अनुपात होता है-
Text Solution
|
- Carbon monoxide (E0) and carbon dioxide (E02) The mass ratio of oxygen...
Text Solution
|
- Carbon and oxygen combine to form two oxides, carbon monoxide and carb...
Text Solution
|
- What is the ratio of carbon and oxygen in carbon monoxide
Text Solution
|