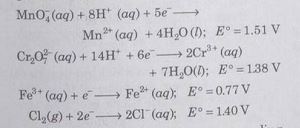A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Standard electrode potential data are useful for understanding the sui...
Text Solution
|
- Calculating the emf from standard potentials: A galvanic cell consis...
Text Solution
|
- Standard electrode potential data are useful for understanding the sui...
Text Solution
|
- Standard electrode potential data are useful for understanding the s...
Text Solution
|
- मानक इलेक्ट्रोड विभव तालिका में दिए गये मानक इलेक्ट्रोड विभवो की सहायत...
Text Solution
|
- मानक इलेक्ट्रोड विभव तालिका में दिए गये मानक इलेक्ट्रोड विभवो की सहायत...
Text Solution
|
- The standard reduction potential of Zn-electrode is -0.76V. The standa...
Text Solution
|
- Standard electrode potential data are useful for understanding the sui...
Text Solution
|
- Standard electrode potential data are useful for understanding the sui...
Text Solution
|