Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
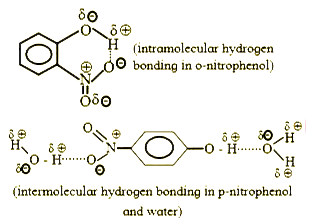
- The solubility ofo-nitrophenol and p-nitrophenol is 0.2 g and 1.7 g/10...
Text Solution
|
- Ortho -nitrophenol is less soluble in water than p-and m-nitrophenols ...
Text Solution
|
- ortho-Nitrophenol is less soluble in water than p and m-nitrophenols b...
Text Solution
|
- Ortho-Nitrophenol is less soluble in water than p- and m- Nitrophenols...
Text Solution
|
- Ortho-Nitrophenol is less soluble in water than p- and m- Nitrophenols...
Text Solution
|
- आर्थों-नाइट्रोफीनॉल, p-तथा m-नाइट्रोफीनॉल की तुलना में जल में कम विलेय...
Text Solution
|
- Ortho -nitrophenol is less soluble in water than p-and m-nitrophenols ...
Text Solution
|
- Ortho-Nitrophenol is less soluble in water than p- and m- Nitrophenols...
Text Solution
|
- Ortho-nitrophenol is less soluble in water than p- and m-nitrophenols ...
Text Solution
|