Text Solution
Verified by Experts
Recommended Questions
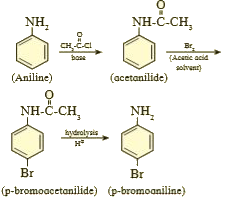
- Write reaction to convert aniline into p-Bromoaniline.
Text Solution
|
- p-bromoaniline is prepared from aniline via
Text Solution
|
- निष्पादित कीजिये --- एनिलीन से p-ब्रोमोएनिलीन
Text Solution
|
- Convert aniline to p-nitro aniline.
Text Solution
|
- एनीलिन से o-ब्रोमोएनिलिन तथा p-ब्रोमोएनिलिन किस प्रकार बनाया जाता है? ...
Text Solution
|
- p-Bromoaniline is formed when aniline is treated with bromine water.
Text Solution
|
- The correct sequence of reactions to be performed to convert benzene i...
Text Solution
|
- How do you convert aniline to parabromo aniline.
Text Solution
|
- Accomplish the following conversions : Aniline to p-bromoaniline
Text Solution
|