Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SRS PUBLICATION-INTEGERS-QUESTION BANK
- Fill in the blanks with suitable integer to make the statement true. ...
Text Solution
|
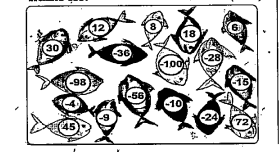
- The fish in the pond below carry some nmbers.choose any 4 pairs and ca...
Text Solution
|
- The fish in the pond below carry some nmbers.choose any 4 pairs and ca...
Text Solution
|
- Calculate the following. (-96) div 16
Text Solution
|
- Calculate the following. 98 div (-49)
Text Solution
|
- Calculate the following. (-51) div 17
Text Solution
|
- Calculate the following. 38 div (-19)
Text Solution
|
- Calculate the following. (-80) div 20
Text Solution
|
- Calculate the following. (-150) div (-25)
Text Solution
|
- Calculate the following. (-600) div 60
Text Solution
|
- Calculate the following. (-54) div 9
Text Solution
|
- Calculate the following. 130 div 65
Text Solution
|
- Calculate the following. (-315) div (315)
Text Solution
|
- The product of two integers is -165. if one number is -15, Find the ot...
Text Solution
|
- Because of covid-19 a company locked down for 6 months and got loss of...
Text Solution
|
- The temperature at 12 noon was 10^@C above zero. If it decreases at th...
Text Solution
|
- A green grocer earns a profit of Rs 7 per kg on tomato and got loss of...
Text Solution
|
- In a class test (+3) marks are given for every correct answer and ( -2...
Text Solution
|
- Write 5 pairs of integers (a,b) such that a div b = - 4. ( Eg : (12...
Text Solution
|
- Identify the laws in the following statements : -3 + 5 = 5 + (-3)
Text Solution
|