Text Solution
Verified by Experts
Topper's Solved these Questions
ACIDS, BASES AND SALTS
OSWAL PUBLICATION|Exercise NCERT EXEMPLAR (Multiple Choice Type Questions )|53 VideosACIDS, BASES AND SALTS
OSWAL PUBLICATION|Exercise Board Corner (Single Answer Type Questions)|18 VideosACIDS, BASES AND SALTS
OSWAL PUBLICATION|Exercise NCERT Corner (Exercise Questions )|4 VideosCARBON AND ITS COMPOUNDS
OSWAL PUBLICATION|Exercise CREATING BASED QUESTIONS|23 Videos
Similar Questions
Explore conceptually related problems
OSWAL PUBLICATION-ACIDS, BASES AND SALTS-NCERT Corner (Exercise Questions ) (Short Answer Type Questions)
- Write word equations and then balanced equations for the reaction , t...
Text Solution
|
- Write word equations and then balanced equations for the reaction taki...
Text Solution
|
- Write word equations and then balanced equations for the reaction taki...
Text Solution
|
- Write word equations and then balanced equations for the reaction taki...
Text Solution
|
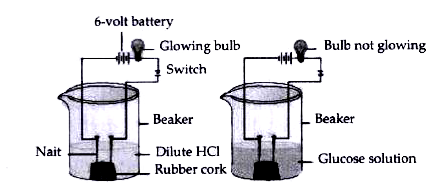
- Compounds such as alcohols and glucose also contain hydrogen but are n...
Text Solution
|
- Why does distilled water not conduct electricity, whereas rain water d...
Text Solution
|
- Why do acids not show acidic behaviour in the absence of water?
Text Solution
|
- Five solutions A,B,C,D and E when tested with universal indicator show...
Text Solution
|
- Equal lengths of magnesium ribbons are taken in test tubes A and B. Hy...
Text Solution
|
- Fresh milk has a pH of 6. How do you think the pH will change as it tu...
Text Solution
|
- A milkman adds a very small amount of baking soda to fresh milk . ...
Text Solution
|
- A milkman adds a very small amount of baking soda to fresh milk. Wh...
Text Solution
|
- Plaster of Paris should be stored in a moisture-proof container. Expla...
Text Solution
|
- What is neutralization reaction? Give two examples.
Text Solution
|
- Give two important uses of washing soda and baking soda.
Text Solution
|